Aluminum anodes have several advantages, such as a lighter weight, and much higher capacity than zinc. However, their electro chemical behavior is not considered as reliable as zinc, and greater care must be taken in how they are used. Aluminum anodes will passivate where chloride concentration is below 1,446 parts per million.
One disadvantage of aluminum is that if it strikes a rusty surface, a large thermite spark may be generated, therefore its use is restricted in tanks where there may be explosive atmospheres and there is a risk of the anode falling.
Aluminum and Zinc anodes are suitable for use in marine environments such as harbors’ and jetties and for internal protection of tanks.
Aluminum is one of the preferred materials to be used as sacrificial anode for carbon steel protection. The efficiency of these can be low due to the formation of oxide layer which passivate the anodes. Currently, to improve its efficiency, there are efforts using a new technique called surface modifications.
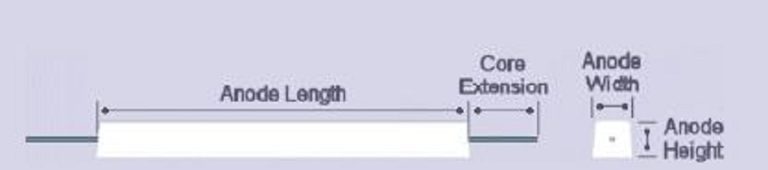
Anode weight, length, width and height dependent upon lifetime and current output.
| ELEMENT | COMPOSITION, % (max.) |
| Silicon | 0.10 |
| Iron | 0.10 |
| Copper | 0.006 |
| Zinc | 3.0 – 5.0 |
| Indium | 0.02 – 0.05 |
| Other elements, each | 0.02 |
| Other elements, total | 0.05 |
| Aluminum | Remainder |
+98(0) 2152826
info@tavana-cps.com
No 39, 14 East St, North Allameh St, Sarv St, Saadat Abad, Tehran - Iran
No 7, Puneh St, Khazar Blvd, Parand Industrial Town, Tehran - Iran
© 2024 Tavana Engineering. Leading the way in keeping pipelines safe and lasting. We focus on advanced protection against rust, special coatings, and detailed inspections. We are also experts in repairing leaks and strengthening pipelines, committed to high-quality service.